SHARE THIS ARTICLE
Professionals
Treating Permanent Dermal Filler Complications
Jun 19, 2019
Introduction
Injectable dermal fillers represent a popular, non-surgical method for the management of the aging face because patients and practitioners consider them as simple and safe. The popularity of minimally invasive cosmetic procedures, specifically the use of hyaluronic acid (HA) fillers, has been driven by a number of factors, including increased effectiveness, affordability, diminished social stigma, and multiple product options. In the United States, the estimated number of injectable filler procedures performed reached 2.3 million in 2017 alone, according to the statistics published by the American Society for Aesthetic Plastic Surgery (ASAPS). Dermal filler treatment, with over 700 million injections, ranked second to botulinum toxin injection.
Most clinicians regard soft tissue augmentation via dermal fillers as versatile and having an impressive safety profile, with the majority of reported adverse effects being mild and self-limiting such as swelling, bruising, redness, and pain. However, increasing use of these fillers has also produced more serious complications and unforeseen events. Some of these complications, although rare, may produce permanent cosmetic injuries or visual deficits. The Food and Drug Administration (FDA) has approved more than 25 soft tissue fillers. It is the injector’s responsibility to understand the properties of each dermal filler product injected. Medical professionals can buy dermal fillers wholesale of almost all the FDA approved brands from Maylips.
Importance of Hyaluronidase
Hyaluronidase is a soluble protein enzyme that catalyzes the degradation of hyaluronic acid, which comprises the most commonly used fillers in the aesthetic market. It is the most valuable agent in treating complications of HA-based fillers. It is cleared by the FDA as a dispersion agent, usually for local anesthetics. However, it is not approved for dissolution of HA and using it to treat complications from facial fillers is considered off-label. Roughly, 30 units of hyaluronidase are needed to dissolve 0.1ml of hyaluronic acid.
Hyaluronidase is generally obtained from three sources: microbes, leech or hookworm, and mammalian testes. The HA-dissolving enzyme has an immediate effect but a short half-life of two minutes with a duration of action of 24-48 hours. Since the product Belotero has more HA cross-linking, it may resolve the slowest and the product Restylane may dissolve the fastest. The use of hyaluronidase in correcting problems with dermal filler injections is allowed provided the patient’s best interest is a respected part of informed consent.
Risks of Permanent Fillers
The popularity of HA fillers may be due to the potential reversibility of HA with hyaluronidase. However, certain cosmetic concerns are best treated by long-lasting fillers that could last up to two to four years. Should complications arise, patients may have to live with them over a long period of time. Non-resorbable fillers that contain polymethylmethacrylate microspheres, hydrogen polymers, or liquid silicone stay indefinitely in tissues.
The decision to use a permanent filling agent should be considered with caution as improper placement of product can lead to long-lasting side effects. Most patients do not understand the difference between temporary and permanent fillers. Patients who seek treatment with permanent fillers must be screened carefully. Those who are unsure of their desired outcomes, who are new to the use of dermal fillers, and have a history of unrealistic expectations with cosmetic procedures, are poor candidates for permanent filler injections.
Complications of Soft Tissue Fillers
All injectable fillers can illicit an unwanted side effect even in the hands of experienced injectors. Good safety data from clinical trials does not necessarily mean you have a risk-free filler at your disposal. Reporting adverse reactions is important, as clinical trials only cover a certain time period, usually ranging from 6-12 months. Complications from dermal filler use are often caused by the use of unapproved products and inexperienced injectors. However, unintentional injuries to anatomic danger zones, such as nerves and blood vessels, can occur even with trained practitioners using FDA-approved products. Vascular compromise associated with dermal filler injection can result in rare but serious complications like blindness, cutaneous ischemic necrosis, and stroke.
In 2018, a multidisciplinary group of experts in aesthetic treatments, published consensus recommendations about the classification of filler complications and their management. The panel proposes to classify complications according to the time of onset (immediate, early, delayed) as suggested by Rohrich et al. and subcategorized into mild, moderate, and severe. Some investigators divide filler complications into four categories: allergic, infective, late-onset, and intravascular events while others prefer non-ischemic versus ischemic categories to represent a more clinically useful perspective.
Immediate Onset (24 hours after procedure)
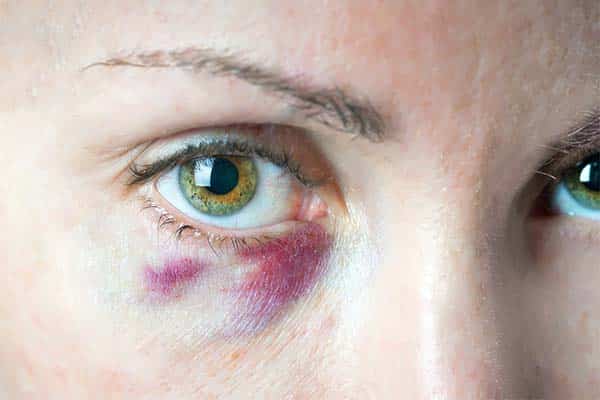
Injection site reactions: are common and predictable side effects that can occur during, immediately following, or hours after filler injection. These include erythema, swelling, and ecchymosis, as well as itching, tenderness, or pain at the injection site. Bruising is more frequently observed after dermal injection into subdermal planes using fanning and threading techniques. Bruising can be reduced by using a cold compress and one can avoid it by injecting the filler slowly. Persistent erythema can be treated with vitamin K cream or rosacea treatments such as oral tetracycline or isotretinoin.
Acute infections: though uncommon, it can appear as acute inflammation or abscesses at the site of injection. A patient may also present with late onset chronic infection. Appropriate antibacterial preparations prior to injections prevent these issues. Late onset infections are often caused by atypical organisms and Mycobacterium. Systemic steroids should not be used in cases of infection but the use of oral antibiotics, including macrolides or tetracycline can be used instead. Herpetic outbreak can occur if the patient has a strong history of perioral herpes simplex virus. To avoid Herpes Simplex Virus (HSV) reactivation, antiviral prophylaxis is recommended at least three days before injection. In patients with active herpes lesion, injections must be postponed until complete resolution of symptoms.
Vascular compromise: must be treated immediately because it can lead to vascular occlusion and tissue necrosis. Occlusion often occurs at the glabella, which is also associated with necrosis with any filler due to limited collateral blood flow in that region. Fillers with larger particle size due to cross-linking lead to a higher likelihood of vascular occlusion. The use of small gauge needles (30-32) and injecting via withdrawal technique can minimize the occurrence of this complication. Avoiding irreversible sequelae lies on the injector’s swift recognition of a vascular event and instigation of necessary treatment. Tissue necrosis is a rare but clinically important dermal filler complication; incidence has been estimated at 0.0011% or 1 in 100,000 cases. Signs of vascular compromise include blanching, bruising, or reticulated erythema during or after injection. Pain and coolness of the skin could also be a sign of impending necrosis. When vascular compromise is suspected, it is vital that the injection is stopped immediately. Attempt aspiration or massage the area to distribute the filler widely. Administer hyaluronidase for HA fillers, and apply 2% nitroglycerin paste to promote vasodilation. Hyaluronidase should be injected at least four hours after the occlusion.
Retinal Arterial Occlusion: exhibits principle symptoms of pain and changes in skin color. In arterial occlusion, pain and whitish vascular reaction are immediate, severe, and disproportionate, whereas venous occlusion is associated with dull or delayed pain. In some cases, there may be absence of pain. Periocular embolism of HA can cause blindness and is associated with excruciating pain. The goal of treatment is to restore retinal circulation within 60 to 90 minutes to prevent irreversible visual loss. Blindness is a devastating complication and the patient should be transferred immediately to the nearest hospital. In 2015, a literature review reported that a higher risk of complications were observed in the glabella, nasal region, nasolabial fold, and forehead. The most common causative material or filler type was autologous fat followed by HA.
Early Onset (24 hours to 4 weeks)
Non-inflammatory nodules: development is common following dermal filler injection. The non-inflammatory type appears when too much filler accumulates in one area, often due to poor injection techniques such as in cases of overcorrection or superficial placement of the filler. In contrast with nodules or granulomas, resulting from inflammatory reaction at the site of infection, non-inflammatory nodules often occur within 24 hours to 4 weeks after injection. If the nodule is caused by accumulation of too much filler material, it can be treated with vigorous massage or administration of hyaluronidase (for HA fillers) to dissolve the filler. The injector may express the filler material out of the dermal tissue through needle aspiration or minimal stab wound incision with evacuation.
Nerve damage: dysesthesia, paresthesia, and anesthesia can occur during an aesthetic procedure. These nerve injuries are often caused by direct trauma, injection of filler into the nerve, and tissue compression. The most commonly affected site is the infraorbital nerve. Injuries to the nerve may be either temporary or irreversible. Although rare, transient Bell’s palsy or marginal mandibular nerve dysfunction has been reported, lasting several weeks. Thorough knowledge of facial anatomy is crucial in minimizing the incidence of such complications. Treatment modalities include a short course of high-dose oral steroids. Antiviral therapy, electrotherapy, physical therapy, and surgical decompression have also been proposed, but with limited evidence of efficacy.
Delayed Onset (more than 4 weeks)
Persistent erythema and telangiectasia: may occur more than 2 weeks after injection. These uncommon complications may be amenable with hyaluronidase. Some cases have also been successfully treated using 532-nm or 1,064-nm laser, either pulsed dye light or potassium titanyl phosphate lasers may help speed up recovery. Prolonged erythema or swelling must be distinguished from delayed hypersensitivity reaction. The latter was theorized to be associated with biofilms.
Tyndall Effect: or Raleigh scattering refers to the bluish hue that is visible within the skin, often observed after HA filler injection. Although the term is a misnomer, the exact mechanism of this effect is yet to be elucidated. Its likelihood is higher in individuals with thinner and lighter skin, but those with greater degrees of skin pigmentation may also develop a darker hue in the periorbital region after HA injection. Hyaluronidase may improve the condition but patients should be counselled that it would also dissolve the filler material and diminish any beneficial effects.
Inflammatory nodules: although more common with permanent fillers, may also develop following administration of HA and collagen. Delayed onset nodules occur within 4 weeks or longer (more than a year) and are usually inflammatory. Causative factors include immune responses to the filler material and/or infection. The use of hyaluronidase, antibiotics, bacteriostatic or immunosuppressive agents (cyclosporine A, hydroxychloroquine, and methotrexate) have been proposed.
Granulomas: are inflammatory papules produced by a group of immune cells called macrophages. Foreign body granulomas or true granulomas form when the body’s immune system tries to ward off substances it perceives as foreign, but fails to eliminate them. Any kind of foreign material can induce the formation of granulomas. This rare side effect is more likely to develop post injection using permanent or semi-permanent fillers. If not treated adequately, granulomas may persist longer and appear more pronounced. Associated swelling and erythema may also be observed. Granulomatous reactions may be treated by hyaluronidase at 150 units per ml.
Biofilms: can cause delayed infections and are much more difficult to treat. Biofilms are aggregate communities of bacteria that surround themselves with secreted polymers to resist phagocytosis. Inflammatory nodules observed after two or more weeks post-injection can be attributed to a biofilm. When biofilm involvement is suspected, diagnostic use of molecular techniques, such as polymerase chain reaction or fluorescence in situ hybridization test (FISH) is recommended, as cultures frequently give negative results even if bacteria is detected in biopsies.
Surgical Removal of Permanent Fillers
Permanent fillers should be reserved for clinicians with extensive experience in soft-tissue augmentation. Moreover, it should not be explored with first time patients as results cannot be reversed and misplaced materials may be impossible to remove completely. When a severe complication occurs, surgical removal of filler particles may be required to resolve problems. When attempting to excise nodules, one must remove all tissue that has been infiltrated, leaving a large defect that will cause marked disfigurement of the face. These attempts are not without associated risks; infective organisms may be introduced during surgery, causing recurrent biofilm infection. One would find it tedious to remove permanent fillers from the complex anatomical regions of the face; in some cases, repeat sessions are necessary for evacuation of residual bulk or correcting facial asymmetry.
Conclusion
The ideal filler provides predictable results, possesses no complications, and allows for reversing if needed. Unfortunately, there is currently no filler in the market with zero risk. Serious debilitating, and irreversible complications can arise from soft tissue augmentation. Prevention is still the best treatment. Potential complications must be openly discussed with patients. The injector should have thorough knowledge of product options and limitations, and strong awareness of the relevant anatomic structures. The majority of adverse effects are avoidable through vigilant practice. Early recognition and treatment are crucial in preventing irreversible damage.
*Disclaimer: Information on Maylips.com is provided for informational purposes only. Self-medication is strictly prohibited. All aesthetic procedures should be provided by the licensed healthcare specialist after the consultation with the personal therapist. The information in this article should not be used for prescribing any medication for the beauty injections.
All brand and medication descriptions in the article are based on the personal opinion and are not endorsed by Maylips.com. The article content was not reviewed for medical validity. Use this article for information and not for a final decision on the procedure.

